The designation “Idiopathic short stature” (ISS) was first used in the 1980s as a description for short children who had normal growth hormone (GH) secretion and otherwise unexplained short stature. ISS acquired clinical significance following the introduction of recombinant human (h)GH in 1985 and the approval of GH deficiency (GHD) by the FDA and EMA for hGH replacement therapy. Trials of hGH in ISS subjects were started, guided by collaborations between pharma companies and academic institutions, because patients with the ISS label were shown to have comparable degrees of short stature to GHD patients.
The designation “Idiopathic short stature” (ISS) was first used in the 1980s as a description for short children who had normal growth hormone (GH) secretion and otherwise unexplained short stature. ISS acquired clinical significance following the introduction of recombinant human (h)GH in 1985 and the approval of GH deficiency (GHD) by the FDA and EMA for hGH replacement therapy. Trials of hGH in ISS subjects were started, guided by collaborations between pharma companies and academic institutions, because patients with the ISS label were shown to have comparable degrees of short stature to GHD patients.
These short children therefore deserved an opportunity to possibly benefit from hGH prescribed in a higher pharmacological dose. Positive data demonstrated efficacy of hGH in the ISS subjects in randomised placebo-controlled trials. Although the growth responses were highly variable, highlighting the heterogeneous nature of these patients, the overall positive responses and absence of safety signals led to successful applications to the FDA and, in 2003, ISS became an approved indication for hGH therapy. Similar applications by pharma companies to the EMA were rejected, largely due to the absence of improvement in quality of life variables. Consequently, ISS is not a reimbursable condition in countries not covered by FDA jurisdictions.
The clinical entity of ISS has acquired scientific respectability, despite the absence of identification of pathogenesis in the majority of patients. Clinicians have continued to use the ISS label for their patients with unexplained short stature and a Consensus Statement on the management of ISS was published in 2008 (1), highlighting the heterogeneity of such patients and giving guidelines for their investigation and management. Patients with familial short stature (FSS), non-familial short stature (NFSS) and constitutional delay of growth and puberty (CDGP) were also included in the definition of ISS.
Since the emergence of molecular biological investigation of patients with growth disorders, which started in the late 1980s and accelerated considerably in the 1990s and through to the 21st century, genetic defects have repeatedly been discovered in children previously labelled as having ISS. Initial genetic studies were mainly in the GH–insulin-like growth factor (IGF)-1 axis, where defects in pituitary development and in genes coding for functional proteins in the pathway of GH action were identified. Examples are monogenic defects in the following genes: GH receptor (GHR); STAT5B; IGF1; IGFALS; IGF1R and PAPPA2 (2). However, recent attention has focused on genes involved in chondrogenesis and growth plate function as a key mechanism regulating normal linear growth (3). Genetic defects in the following growth plate genes have been identified in children carrying the label of ISS: SHOX; ACAN; NPR2; IHH; FGFR3.
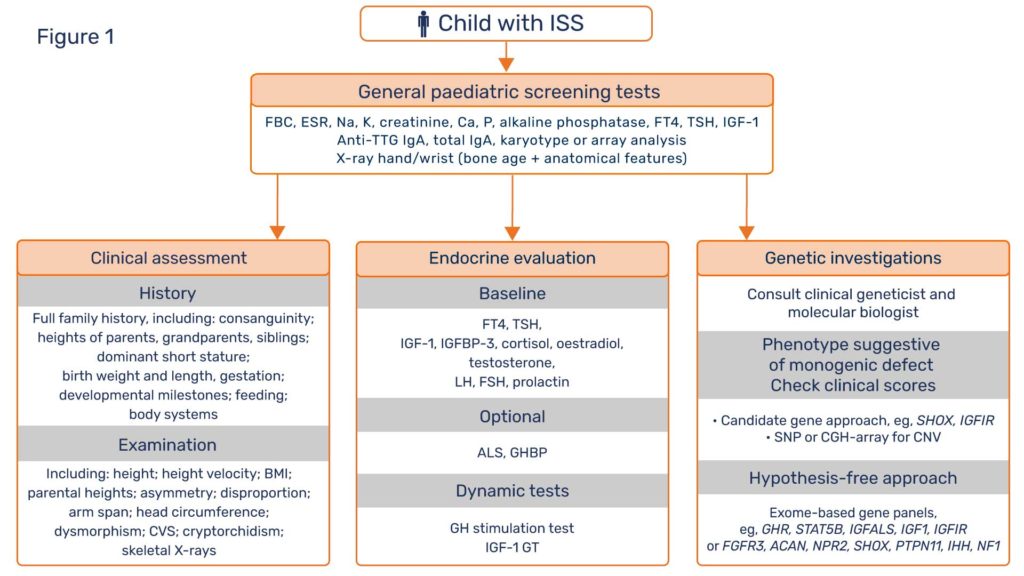
In view of these recent discoveries, patients labelled as ISS deserve further investigation in an attempt to define their true pathogenesis. “ISS” is not a diagnosis, but a statement of diagnostic uncertainty. It is now clear that the three disciplines of diagnostic assessment, namely clinical evaluation, endocrine characterisation and genetic investigation, need to have equal status in the hierarchy of the short stature protocol (Figure 1). Clinical assessment is of crucial importance with essential documentation of family history, parental, grandparental and sibling height data, dysmorphology and body disproportion examination, in addition to developmental, feeding and behavioural milestones (4). General paediatric disorders, such as celiac disease and Turner syndrome need to be excluded. Endocrine evaluation should document GH and IGF-1 status. Genetic investigations should centre around the search for monogenic defects with major growth effects, rather than polygenic defects with multiple minor effects.A working relationship should be established between the clinician and a molecular biologist for discussion of which short patients should undergo genetic tests, which are the appropriate techniques to use and how can the results of the tests be best interpreted. Such a relationship will bring considerable dividends in terms of academic learning, patient care and research opportunities. For example, candidate gene sequencing, indicated when a phenotype suggests a specific genetic diagnosis, is largely being replaced by the hypothesis-free approach of whole-exome sequencing using specific gene panels, which have given diagnostic yields in ~35% of unexplained short stature patients in some reports (5).
ISS is a convenient term for patients with unexplained short stature and is likely to remain in clinical usage. The scheme proposed in Figure 1 gives equality to the three diagnostic disciplines of clinical, endocrine and genetic studies to pursue the primary cause of short stature in unexplained cases. This approach has the realistic chance of clarifying the molecular pathogenesis of known or novel growth disorders. By explaining the basic mechanistic defects, genetic results can guide therapy and thereby enhance progression to optimisation of clinical management.