
Find out about the risks and management of endocrine adverse events in children and adolescents undergoing immune checkpoint therapy for cancer.
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target regulatory molecules on T cells to enhance immune responses against cancer, primarily cytotoxic T-lymphocyte associated protein (CTLA)-4, PD-1, and PD-L1 (Figure 1) [1, 2]. These checkpoints normally serve to regulate immune activation and prevent autoimmunity. By blocking them, ICIs release the brakes on T cells, allowing them to recognise and attack tumour cells. This immunological strategy has revolutionised cancer therapy, especially in malignancies where conventional treatments have failed. The first ICI, ipilimumab, targeting CTLA-4, was approved in 2011 for metastatic melanoma, and since then, multiple agents have gained approval for use in both adults and children [3].
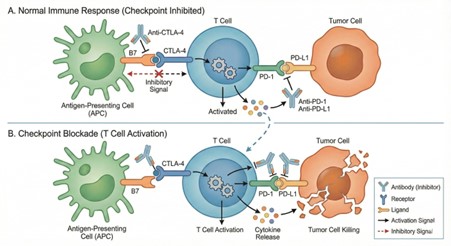
Figure 1: Mechanism of immune checkpoint inhibitors. A. Normal immune response: T cell activation is restrained by inhibitory signals mediated through CTLA-4 binding to B7 and PD-1 interaction with PD-L1, limiting cytokine release and tumour cell killing. B. Checkpoint inhibitor blockade: Antibodies targeting CTLA-4, PD-1, or PD-L1 disrupt these inhibitory pathways, restoring T cell activation, cytokine release, and effective tumour cell killing.
Schematic generated with assistance from the AI tool Gemini and subsequently edited by the author.
ICIs are now integral to the treatment of a wide range of cancers. In adults, they are approved for melanoma, non-small-cell lung cancer, renal cell carcinoma, hepatocellular carcinoma, and many others [1, 2]. Their use has expanded to adjuvant and neoadjuvant settings, and they are often combined with chemotherapy or targeted therapies. In paediatric populations, ICIs are primarily used within clinical trials for relapsed or refractory cancers [3]. Although still in early-phase studies, ICIs have shown efficacy in paediatric melanoma, Hodgkin lymphoma and certain central nervous system tumours, and are being explored for SMARCB1-deficient cancers. Their mechanism offers a novel approach against tumours that evade immune detection (Figure). However, the same immune activation that underpins their success also leads to a wide spectrum of immune-related adverse events (irAEs) [4].
Unlike traditional chemotherapy, which causes direct cytotoxicity, ICIs provoke immune-mediated inflammation [4]. irAEs can affect any organ system, with dermatological, gastrointestinal, hepatic, pulmonary, and endocrine toxicities being most common. These events typically occur within weeks to months of initiating therapy but can also emerge later or persist long after treatment cessation. Severity ranges from mild to life threatening, and symptoms may mimic cancer progression or other treatment effects, complicating diagnosis. Importantly, irAEs may correlate with better oncological outcomes, suggesting that immune activation against tumours and self-tissues may share mechanistic pathways. Nonetheless, irAEs require prompt recognition and management to prevent morbidity [5].
Endocrine irAEs are among the most frequent and persistent complications of ICI therapy [6, 7]. They often result in irreversible glandular damage, necessitating lifelong hormone replacement. Thyroid dysfunction is the most common endocrine irAE, typically presenting as transient thyrotoxicosis followed by hypothyroidism. In paediatric patients, hypothyroidism occurs in up to 20% and hyperthyroidism in up to 15%, often within weeks of treatment initiation. The presence of thyroid autoantibodies during the thyrotoxic phase increases the risk of progression to hypothyroidism. Levothyroxine therapy is effective, and ICI treatment is usually continued [8].
Hypophysitis, an inflammation of the pituitary gland, is particularly associated with anti-CTLA-4 agents and may lead to multiple pituitary hormone deficiency. Central adrenal insufficiency resulting from hypophysitis is often permanent. Pituitary MRI may show gland enlargement, but imaging findings are variable. In children, hypophysitis is rare but can have significant implications for growth and pubertal development. Hormone replacement therapy is essential and should be monitored regularly [8].
Primary adrenal insufficiency is less common but potentially life threatening. It occurs in 1–2% of adults receiving single-agent ICIs and up to 9% with combination therapy. Paediatric cases may present earlier and more acutely than adult cases The mechanism involves immune-mediated destruction of the adrenal cortex, possibly via anti-adrenal antibodies. Symptoms such as fatigue may be nonspecific, underscoring the need for biochemical surveillance. Morning cortisol and adrenocorticotropic hormone levels are critical for diagnosis, and hydrocortisone replacement should be initiated promptly. An emergency card is recommended for patients at risk [7].
ICI-induced diabetes mellitus is another serious irAE, particularly in children. The incidence ranges from 0.5–2% in adults to 1–9% in paediatric patients. It often presents abruptly with diabetic ketoacidosis and is characterised by high, low or undetectable C-peptide levels. Interestingly, about half of low–undetectable C-peptide cases are antibody-negative, suggesting a distinct pathophysiology from type 1 diabetes. Genetic predisposition and combination ICI therapy are risk factors. Insulin therapy is required, and long-term monitoring is essential [7, 8].
Other endocrine irAEs include hypoparathyroidism and gonadal dysfunction, though these are less well characterised.
In paediatric and adolescent patients, endocrine irAEs resemble those seen in adults but carry distinct developmental implications [8, 9, 10]. Thyroid dysfunction and diabetes are more frequently observed, while hypophysitis and adrenal insufficiency remain rare but clinically significant. The implications for growth, puberty, and long-term development are profound. Children may be more susceptible to lasting hormonal deficits due to the sensitivity of maturing endocrine systems. Hormone replacement therapies are generally effective, but the balance between managing irAEs and maintaining cancer control requires careful navigation in this age group.
ICIs have reshaped cancer therapy, offering renewed hope for patients with advanced or refractory malignancies. Yet, their capacity to trigger autoimmune endocrine complications presents ongoing challenges, especially in paediatric populations. These irAEs are frequently irreversible but manageable, and they rarely necessitate discontinuation of cancer treatment when therapeutic benefit is evident. Optimising outcomes requires a multidisciplinary approach integrating routine hormonal surveillance, timely initiation of replacement therapies and clear patient education.
In children and adolescents, the developmental impact of endocrine irAEs demands particular attention [9, 10]. The European Society for Pediatric Endocrinology advocates for age-specific monitoring strategies and collaborative care models that reflect the unique vulnerabilities of growing patients [8]. As most current protocols are derived from adult data, there is a need for paediatric-focused research to address gaps in knowledge concerning long-term outcomes, diagnostic standards, and predictive biomarkers.

