Case study introduction
A 12-year-old boy with a diagnosis of Carney Complex (CC) has accelerated growth. How does the endocrinologist manage this patient?
Please read more of the patient presentation and family history below and answer the questions.
- Patient referred to endocrinology age 4 months following a genetic diagnosis of CC due to a change in the PRKAR1A gene. He had inherited the gene variant from his father who had a family history of multiple atrial myxomas.
- CC is a very rare multiple neoplasia syndrome with an unknown incidence.
- CC is characterised by pigmented lesions of the skin, myxomas in cardiac and cutaneous tissues, and several other endocrine and non-endocrine neoplasms.1,2
1. What investigations would you like to obtain on this patient, and how frequently?
Screening for development of endocrine neoplasms is vital as tumours can develop at any time.2,3 Therefore, the below protocol is suggested:
Investigation | Frequency (months) |
Pituitary hormone blood tests: cortisol, insulin-like growth factor (IGF)-1, testosterone, thyroid function tests (TFTs), gonadotrophins, prolactin. | 6-12 |
Pubertal staging | 6 |
Height measurements | 6 |
Testicular ultrasound | 12 |
Thyroid and adrenal ultrasound | Baseline and as necessary |
Pituitary magnetic resonance imaging (MRI) | If clinical suspicion of adenoma |
Echocardiograms | 6 |
Ophthalmology review | Baseline and as necessary |
- Two atrial myxomas excised at age 3 and 8 years.
- Testicular ultrasound at 8 years demonstrated small areas of calcification consistent with Sertoli cell changes.
- All other blood tests and ultrasound scans were normal.
- At age 10 years his IGF-1 level was raised:
| DATE | IGF-1 (normal range [NR] 8.9–41.1 nmol/L) |
| MAY 2020 | 46.4 nmol/L |
| OCTOBER 2020 | 49.5 nmol/L |
Auxology as below:
- Height 152.2cm. + 1.97 standard deviation score (SDS).
- Father’s height 185.4 cm. Mother’s height 182.7 cm. Mid-parental height 182.67 cm. Mid parental height +0.84 SDS
- Height velocity 5.9 cm/year
- Pubertal status age 10.31 years – Tanner stages: axillary hair 1, pubic hair 1, genitalia 2. Testicular volumes 8 mL bilaterally. It is important to note here that there is difficulty determining true testicular development in patients with testicular tumours or calcifications. Therefore, testicular ultrasound was used to confirm that the volume of testes was not due to testicular tumours but due to gonadotrophin-dependent activation of the pituitary–gonadal axis.
- Tanner-Whitehouse (TW)2 bone age 10.38 years. Chronological age 10.31 years
2. Are you concerned about this raised IGF-1?
- This patient was entering puberty, within mid parental height and had normal height velocity for his age and bone age.
- Our hospital normal lab values for IGF-1 in children, aged 11+ years is 19.6–65.8 nmol/L, therefore his raised IGF-1 levels were appropriate for a boy in puberty.
- This raises less concern over raised IGF-1, however, in the context of CC, a pituitary MRI was arranged and this was normal.
INCREASING HEIGHT VELOCITY
The patient continued to grow quickly and at 11.1 years auxology was:
- Height 168.4 cm. +2.8 SDS.
- Height velocity 9.5 cm/year

However, IGF-1 remained in the normal range:
| Month | IGF-1 (NR 18.6–65.8 nmol/L) |
| March 2022 | 55.5 nmol/L |
| April 2022 | 60.2 nmol/L |
- A second pituitary MRI was performed and identified a pituitary lesion thought to be consistent with a growth hormone (GH) secreting adenoma.

- A GH suppression test was performed which confirmed GH excess (peak GH level 5.02 µg/L)
In this instance, it was clinical concern regarding rapid growth velocity that instigated the pituitary MRI. This demonstrates the importance of accurate and regular measurement of height in this patient cohort, and how quickly tumours can develop in patients with CC.
3. What monitoring should you arrange for this patient given this new finding?
Patients with GH excess can develop acromegaly and gigantism, some of the clinical manifestations of which include visceromegaly, cardiac hypertrophy, hypertension, insulin resistance, sleep apnoea and macroglossia. Although these symptoms can develop slowly over the years, they are associated with increased morbidity and reduced life expectancy, and therefore careful management and monitoring of patients is important to improve outcomes.4
Therefore, the investigations detailed below were arranged:
- Sleep study – normal.
- Repeat assessment of other pituitary hormones – normal.
- Neurosurgical review.
4. When considering treatment for GH excess which of the below treatments might you consider and why?
- Watch and wait
- Surgical intervention
- Medication such as somatostatin analogues
- Seek the advice of an adult endocrinologist
SURGICAL INTERVENTION
- Neurosurgical review demonstrated that surgical excision of the adenoma was possible.
- Surgery could cause further pituitary hormone deficiencies.
- Surgery may not cure the patient as there is likely to be diffuse involvement of the pituitary.
- Patient has already had two cardiac surgeries to remove atrial myxomas. The trauma that major surgery can have on patients and families should not be understated. At this point both patient and family wanted to avoid further surgery.
WATCH AND WAIT
- At this time this patient was not suffering any medical complications of GH excess.
- Consensus amongst all professionals in the Liverpool Pituitary multidisciplinary team was to watch and wait.
Therefore, we arranged:
- Regular ophthalmology review to assess if adenoma was impacting visual pathways.
- Regular surveillance MRI scans to track growth of adenoma.
- Regular monitoring of pituitary hormones and IGF-1.
STARTING A SOMATOSTATIN ANOLOGUE – LANREOTIDE
The patient continued to be monitored over the course of a year:
- IGF-1 levels and height increased.
- Height velocity – 11.19 cm/year at age 12 years 8 months.
- Pubertal status – Tanner Stages axillary hair 1, pubic hair 1, genitalia 2. Testicular volumes 10 mL.
- TW2 bone age 14.4 years. Chronological age 12.8 years
|
DATE |
GH VALUE |
RANGE |
HEIGHT |
HEIGHT SDS |
|
OCTOBER 2022 |
63.6 nmol/L |
18.6–65.8 |
177.1 cm |
+3.13 |
|
FEBRUARY 2023 |
66.8 nmol/L |
18.6–65.8 |
180.0 cm |
+3.21 |
|
MARCH 2023 |
69.8 nmol/L |
18.6–65.8 |
Not taken |
Not taken |
- Rapid growth is to be expected during a pubertal growth spurt in a patient with the bone age and testicular volumes above.
- In the context of a pituitary adenoma and raised IGF-1 the decision was made to start treatment with a somatostatin analogue to prevent complications of GH excess.
- This medication is not licensed in paediatric patients and advice was sought from adult clinicians more experienced in the treatment of GH excess.
LANREOTIDE:
- Lanreotide is a long-acting analogue of somatostatin which binds to human somatostatin receptors 2 and 5 to inhibit GH secretion.
- Lanreotide is the first-line medical treatment for GH excess.5
- Administered as a depot subcutaneous injection once a month.
Lanreotide can cause the below side effects6:
- Abdominal pain
- Nausea and vomiting
- Diarrhoea
- Cholelithiasis
- Dysglycaemia
Our patient started treatment at a dose of 60 mg every 4 weeks (adult dosing range 60–120 mg/month).
5. What would you like to monitor going forward?
- Height measurements
- IGF-1 during treatment
- Glycated haemogobin (HbA1c)
- Side effects
- MRI scans to monitor size of adenoma.
PATIENT HISTORY DURING LANREOTIDE TREATMENT
- Patient reviewed by paediatric endocrine nurse specialists once a month for height measurements, blood tests and lanreotide injection.
- Patient suffered abdominal cramps and diarrhoea when he started treatment and after dose increases. Dose was occasionally reduced to help with side effects and loperamide also used to alleviate symptoms. Side effects settled over time.
- Over a period of 10 months the monthly dose was increased from 60 mg to 90 mg and then 120 mg.
- Size of adenoma reduced from 14 mm x 18 mm x 15 mm to 10 mm x 14 mm x 6 mm.
- IGF-1 remained elevated despite dose increases, however his growth stopped, and his epiphyses looked mature on X-ray.
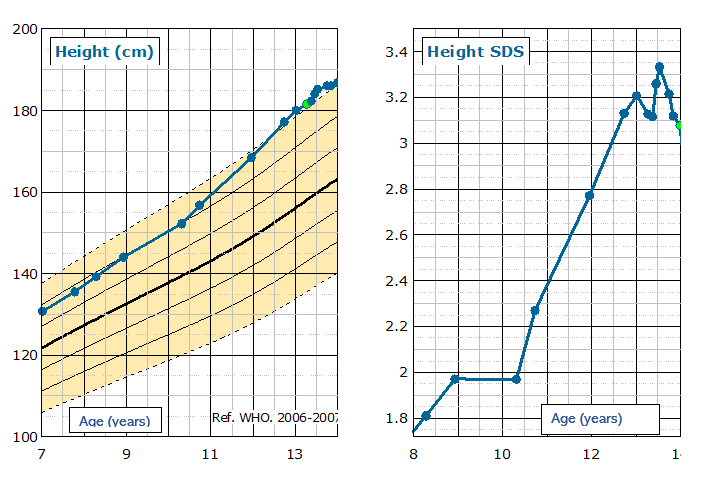
Table 3: IGF-1 and growth response to treatment with lanreotide
| Months after the start of treatment | IGF-1 (NR 18.6–65.8 nmol/L) | DOSE OF LANREOTIDE (mg) | HEIGHT (cm) | HEIGHT SDS |
| Baseline | X | 60 | X | X |
| 1 | 70.2 | 40 | 181.5 | 3.13 |
| 2 | 67.0 | 60 | 182.3 | 3.12 |
| 3 | 61.2 | 60 | 184.0 | 3.26 |
| 4 | 69.3 | 60 | 185.2 | 3.33 |
| 5 | 73.3 | 90 | X | X |
| 6 | 72.4 | 90 | X | X |
| 7 | 75.4 | 120 | 186.0 | 3.21 |
| 8 | 73.5 | 120 | 186.0 | 3.12 |
| JANUARY 2024 | 78.6 | 120 | 186 | 3.08 |
ALTERNATIVE TREATMENT TO SOMATOSTATIN ANALOGUES
- Treatment with lanreotide halted progression of the size of the pituitary adenoma, but IGF-1 levels remained raised.
- Surgical excision and medical treatment options were discussed again by the Liverpool Pituitary multidisciplinary team.
- Based on these discussions and the family’s preference, a decision was made to start alternative medical therapy in the form of a GH receptor antagonist.
GROWTH HORMONE ANTAGONIST – PEGVISOMANT
- Pegvisomant is GH antagonist which binds with GH receptors, blocking GH binding and interfering with intracellular GH signal transduction.
- It is given subcutaneously once daily and is licensed in adults who are prescribed a loading dose of 80 mg, followed by 10 mg daily increasing in 5-mg increments according to IGF-1 response to a maximum dose of 30 mg/day.6,7
Pegvisomant can have the below side effects7:
- Deranged liver function tests (LFTs)
- Lipodystrophy
- Arthralgia
This patient was treated with lanreotide 120 mg monthly whilst hospital and pharmacy approval and funding for pegvisomant was obtained.
- At age 14 years and 4 months he received a loading dose of pegvisomant 80 mg and began daily doses of 10 mg. Lanreotide therapy was discontinued.
6. What would you like to monitor going forward?
- Height measurements
- IGF-1 during treatment
- LFTs
- Continue monitoring pituitary hormones as the adenoma could cause other pituitary dysfunctions
- MRI monitoring of adenoma.
PATIENT TODAY
- Patient and family trained on administration of pegvisomant by a paediatric endocrine nurse specialist, and he now self-administers all injections.
- Currently patient has had no side effects of pegvisomant demonstrated in biochemistry or physical examination.
- IGF-1 levels demonstrated good initial response to pegvisomant.
- When IGF-1 level was noted to have increased, the dose of pegvisomant was increased to 15 mg/day as per adult guidance (Table 4)
Table 4: IGF-1 response to the introduction of pegvisomant
| Months after the start of medical treatment | IGF-1 (NR 18.6–65.8 nmol/L) | TREATMENT |
| 12 | 89.2 | lanreotide 120 mg/day |
| 13 | 85.8 | lanreotide 120 mg/day |
| 14 | 74.8 | pegvisomant 10 mg/day |
| 15 | 62.4 | pegvisomant 10 mg/day |
| 16 | 71.4 | pegvisomant 15 mg/day |
| 17 | 61.2 | pegivsomant 15 mg/day |
- Patient’s height is static and growth almost complete.
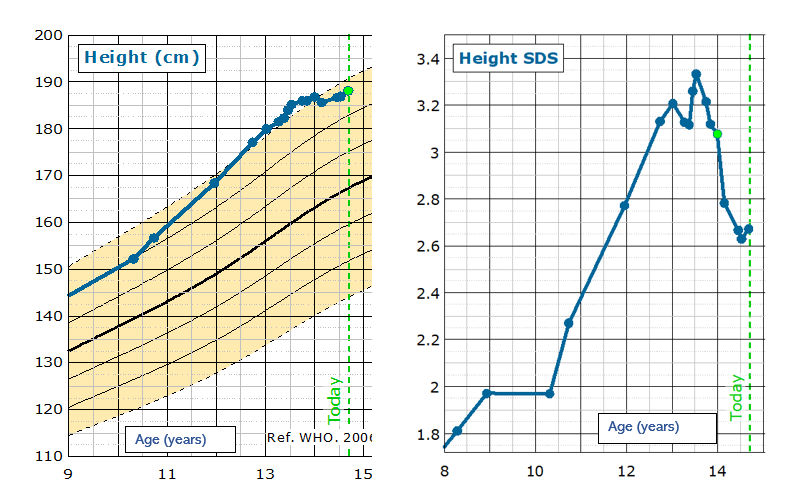
- Patient additionally developed secondary adrenal insufficiency (peak cortisol of 348 nmol/L on synacthen testing) and secondary hypothyroidism. He was started on hydrocortisone and levothyroxine which continues to be monitored by the endocrinologist.
- He continues to be monitored with height, weight, bloods and blood pressure checked by paediatric endocrine nurse specialists on a monthly basis.
- His dose of pegvisomant may continue to be increased if IGF-1 levels rise.
CONCLUSION
CC is a rare genetic condition that can be incredibly complex to manage. This case study highlights this and the importance of regular screening and review.
It also highlights the many factors to consider when treating a patient with GH secreting pituitary adenoma, and the benefit of exploring medications that are not usually prescribed in a paediatric setting. Particularly in patients where surgical involvement is not possible or wanted by the family.
Finally, this case study emphasises the value of communication between professionals, notably between paediatric and adult endocrinologists and between the members of multidisciplinary team who must work together, sharing information, co-ordinating appointments, and obtaining approval for use of these medications in the best interest of the patient.
ACKNOWLEDGEMENTS
Many thanks to Professor Joanne Blair for her support and encouragement with this case study.
- Correa R., Salpea P, Stratakis CA. Carney complex: an updateEur J Endocrinol 2015;173: M85–M97 https://academic.oup.com/ejendo/article-abstract/173/4/M85/6660773
- Eunice Kennedy Shriver National Institute of Child Health and Human Development Information Resource Center. Carney Complex. Accessed online 23.10.2024 https://www.nichd.nih.gov/health/topics/factsheets/carneycomplex
- Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney Complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab 2001; 86: 4041–4046 https://academic.oup.com/jcem/article-abstract/86/9/4041/2848323
- Lugo G, Pena L, Cordido F. Clinical Manifestations and Diagnosis of Acromegaly. Int J Endocrinol 2012; 1 February https://onlinelibrary.wiley.com/doi/10.1155/2012/540398
- Paisley AN,Trainer PJ.Medical treatment in acromegaly. Curr Opin Pharmacol 2003; 3: 672–677 https://www.sciencedirect.com/science/article/pii/S1471489203001656
- Zahr R, Fleseriu M. Updates in Diagnosis and Treatment of Acromegaly. Eur Endocrinol; 14:57–61 https://pmc.ncbi.nlm.nih.gov/articles/PMC6182922/
- Tritos NA, Biller BMK. Pegvisomant: a growth hormone receptor antagonist used in the treatment of acromegaly. Pituitary 2016; 20:129–135 https://link.springer.com/article/10.1007/s11102-016-0753-y
Graphs created using https://www.growthxp.com/
WHO child growth standards: growth velocity based on weight, length and head circumference: methods and development. World Health Organization 2009: https://www.who.int/publications/i/item/9789241547635
Licence: CC BY-NC-SA 3.0 IGO.


